Limitin Lamivu Zidovu Tablet Anti HIV
Basic Info
Model No.: A1609-1201A
Product Description
Model NO.: A1609-1201A
Usage Mode: For oral administration
State: Liquid
Type: Biological Products
Trademark: GUYENNE
Origin: France
Application: Internal Medicine
Suitable for: All
Shape: Capsules
Pharmaceutical Technology: Chemical Synthesis
Specification: 300mg
HS Code: 300290309
Tablet for Anti HIV
| Specification | 300mg+150mg |
| Package | 30tablets/bottle |
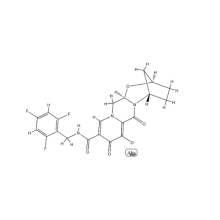
Tenofovir disoproxil is an antiretroviral medication used to prevent and treat HIV/AIDS and to treat chronic hepatitis B. The active substance is tenofovir, while tenofovir disoproxil is a prodrug that is used because of its better absorption in the gut.
Medical uses
Tenofovir disoproxil fumarate (tenofovir DF) is a bioavailable prodrug of tenofovir, a potent nucleotide analogue reverse-transcriptase inhibitor with activity against human immunodeficiency virus (HIV) and hepatitis B virus. It is administered as a single 300-mg tablet once daily. It was approved for the treatment of HIV infection on the basis of data from clinical trials demonstrating activity in treatment-experienced patients, and it was subsequently shown to be effective when used as a component of initial therapy. Tenofovir DF is active against some nucleoside-resistant strains of HIV. However, cross-resistance is associated with multiple thymidine analogue mutations that include 41L or 210W. The signature mutation is the K65R mutation, which causes variable loss in susceptibility to tenofovir DF, . Tenofovir DF has been well tolerated in clinical trials with durations of follow-up up to 96 weeks. It is associated with more-favorable lipid profiles than and has not been associated with the mitochondrial toxicity attributed to other nucleoside analogues.
Tenofovir disoproxil fumarate (tenofovir DF) is an orally bioavailable prodrug of tenofovir. It is the first nucleotide analogue reverse-transcriptase inhibitor (NtRTI) to have been approved by the US Food and Drug Administration (FDA) for the treatment of HIV infection. Since its approval in October 2001, this analogue of adenine 5′ monophosphate has quickly become a widely used component of antiretroviral regimens for both treatment-naive and -experienced patients on the basis of its efficacy and tolerability in several clinical trials. Contact us if you need more details on Tenofovir. We are ready to answer your questions on packaging, logistics, certification or any other aspects about Disoproxil、Fumarate. If these products fail to match your need, please contact us and we would like to provide relevant information.
Product Categories : Anti-Hiv Drug
Premium Related Products
Other Products
Hot Products
High Quality 200mg Amiodarone Hydrochloride TabletsLarge Stock Whiten Skin Monobenzone CreamGeneral Medicine Omeprazole 20mg Injection for Gastrohelcosis and Stomach AcidGeneral Medicine Ceftriaxone Sodium InjectionReady Stock Skin Whitening Anti-Aging Vitamin C InjectionOEM Tablet 500mg ParacetamolHigh Quality 500mg Hard Capsules Amoxicillin (amoxycillin)Best and Low Price Ceftriaxone Sodium Injection CeftriaxoneBest Quality Omega 3 Deep Sea Softgel Capsule Fish OilFDA Approved Curative Antimalarial ArtemisininMedicine Treating Brain Injury Citicoline Sodium InjectionProtease Alpha Chymolase for Inflammatory EdemaGeneral Medicine Medroxyprogesterone Acetate InjectionAmikacin Injection General Medicine DrugsSkin Whitening Gsh Personal Care Glutathione InjectionBody Slimming Fitness Lose Weight Weight Loss L-Carnitine Injection2.0g/5ml