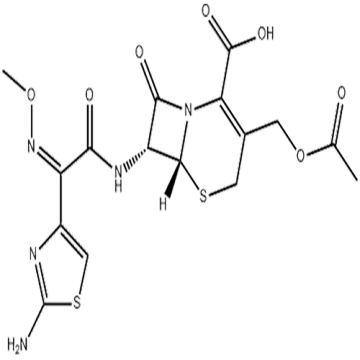
Semigenerative Antibiotics Roxithromycin Cas 80214-83-1
| Payment Type: | L/C,T/T,D/P |
|---|---|
| Terms of Trade: | FOB,CFR,CIF,CPT,CIP |
| Delivery Time: | 10 Days |
| Packaging: | 200L/PE Drum |
|---|---|
| Productivity: | 2000MT/A |
| Brand: | YFL |
| Transportation: | Ocean,Air |
| Place of Origin: | Shandong Prov. |
| Supply Ability: | 2000MT/A |
| Port: | Shanghai,Beijing,Qingdao |
Click on the follow link to find out more information: http://www.yfluorine.com/new-chemical-materials/
Company Info
- Company Name: Shandong YunFengLian Chemical Co.,Ltd
- Representative: Ms,Zhu
- Product/Service: Ethyl Bromodifluoroacetate , Ethy 2-fluoroacetoacetate , 2 2-Difluoroethanol , Chlorodifluoroacetic Acid , Ethyl Chlorodifluoroacetate , 2 2 2-Trifluoroacetamide
- Capital (Million US $): 5000,000RMB
- Year Established: 2020
- Total Annual Sales Volume (Million US $): Above US$100 Million
- Export Percentage: 91% - 100%
- Total Annual Purchase Volume (Million US $): Above US$100 Million
- No. of Production Lines: Above 20
- No. of R&D Staff: 21 -30 People
- No. of QC Staff: 21 -30 People
- OEM Services Provided: yes
- Factory Size (Sq.meters): 5,000-10,000 square meters
- Factory Location: ZhongRun Centry Center,No.12111,JingShi Road,LiXia District
- Contact Person: Ms. Bonnie
- Tel: +86-531-88287585
Premium Related Products
Other Products
Hot Products
High Quality 200mg Amiodarone Hydrochloride TabletsLarge Stock Whiten Skin Monobenzone CreamGeneral Medicine Omeprazole 20mg Injection for Gastrohelcosis and Stomach AcidGeneral Medicine Ceftriaxone Sodium InjectionReady Stock Skin Whitening Anti-Aging Vitamin C InjectionOEM Tablet 500mg ParacetamolHigh Quality 500mg Hard Capsules Amoxicillin (amoxycillin)Best and Low Price Ceftriaxone Sodium Injection CeftriaxoneBest Quality Omega 3 Deep Sea Softgel Capsule Fish OilFDA Approved Curative Antimalarial ArtemisininMedicine Treating Brain Injury Citicoline Sodium InjectionProtease Alpha Chymolase for Inflammatory EdemaGeneral Medicine Medroxyprogesterone Acetate InjectionAmikacin Injection General Medicine DrugsSkin Whitening Gsh Personal Care Glutathione InjectionBody Slimming Fitness Lose Weight Weight Loss L-Carnitine Injection2.0g/5ml